Hokkaido University Hospital
Clinical Research and
Medical Innovation Center
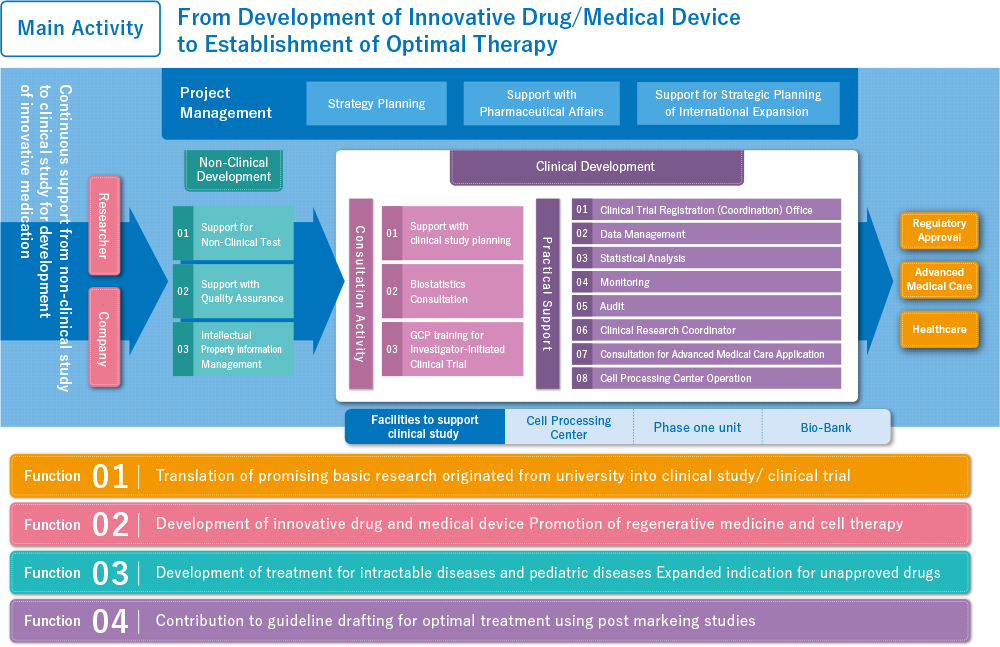
The operating goal of the Hokkaido University Hospital Clinical Research and Medical Innovation Center is to actualize optimal medical treatments. To this end, we are involved in creating items such as ground breaking pharmacological products and medical equipment developed in Japan and designing treatment methodologies for patients with intractable conditions. We also support research projects for the purpose of ensuring the implementation of optimal clinical research. To achieve these goals, we have assembled a staff of medical technology development and clinical research specialists and operate cutting edge facilities such as the Cell Processing Room and the Biobank.
Together with not only our doctors and researchers, but also employees at partner companies and, most of all, all of the patients and test subjects who participate in our studies, we will keep striving to actualize the creation of innovative medical technologies and the optimal implementation of clinical research.
1Center activity summary
2Organization Chart
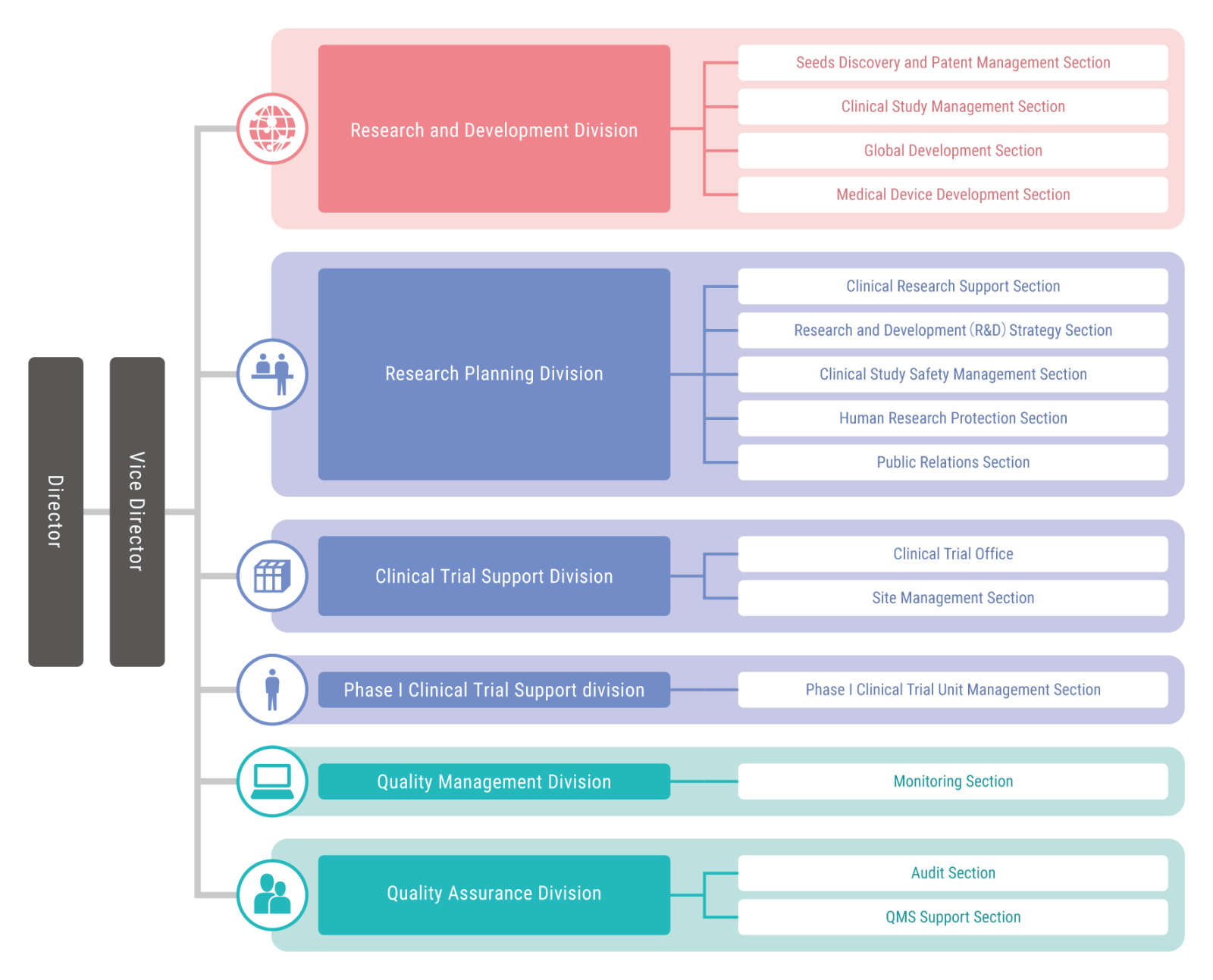
3Divisions
- Clinical Research Operations Division
- Provides support for the development of clinical research that aims to excavate, cultivate, and commercialize seeds for the development of medical treatment technology
- Research Planning Division
- Providing comprehensive support by utilizing ARO functions from consultation to the start of research implementation support
- Clinical Trial Support Division
- Provides support for the smooth execution of clinical trials and support that facilitates a smooth clinical trial process for subjects in the hospital environment
- Phase I Clinical Trial Support division
- Using the Phase I Unit, this Division executes early stage investigatory clinical research and clinical trials targeting healthy adults and promotes clinical research on promising seeds from the university, etc.
- Quality Management Division
- Verifies the appropriateness of and provides quality control for execution in clinical trials and other forms of clinical research through source document verification
- Quality Assurance Division
- Implements quality assurance activities designed to improve clinical research reliability
- Planning and Management Division
- Provides the clerical support necessary for Center activities to proceed smoothly
4Access
Kita 14, Nishi 5, Kita-ku, Sapporo, Hokkaido, 060-8648 Japan
TEL: +81-11-706-7061 FAX: +81-11-706-7977